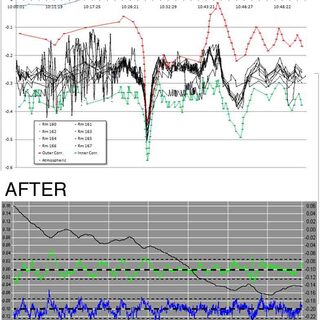
Addressing Biosafety Regulations with Risk Assessment and Verification Planning
Using a verification planning process to meet current regulations—for directional airflow, atmospheric pressure reference, containment barriers, and other common compliance measures—with solutions based on risk analysis and standard operating procedures (SOPs), takes a project through planning and design to fully licensed and operational, and helps minimize spending on solutions to a level that is risk-appropriate.