Individually ventilated cage (IVC) systems are the standard for housing laboratory rodents, and, while research publications commonly refer to the use of IVCs in rodent-based studies, there are considerable differences in their mechanical design and operation that make a generalized, catch-all term problematic. This ambiguity has significant implications in certain research fields for ensuring consistency and the need to reproduce findings. As a result, a growing number of researchers are proposing inclusion of key details in the materials and methods section of published manuscripts to clarify the wide array of available IVC technologies, operating configurations, and performance variables.
The use of IVCs for rodent-based experiments offers many benefits, including the prevention of cross-contamination. Since most IVCs use low velocity HEPA filtration, they also improve animal air quality by removing waste gases—such as ammonia and CO2—and have been shown to decrease allergen exposure for lab personnel, especially if their exhaust is connected to the facility’s main exhaust system. But there are many different cage types and rack configurations that each create different living environments for the rodents.
“Scientific literature generalizes IVCs as a single term, but not all IVCs function the same way,” explains Scott Perkins, senior director of Comparative Medicine Services at Tufts University. “One type of IVC may perform very differently than another type, but rarely is the system described in sufficient detail in the manuscripts.”
One potential disadvantage that comes with IVCs is the fact that higher air velocities can cause stress in animals that results in depressive behavior, impacts cognition, and increases sensitivity to the locomotor stimulation of specific drug treatments. This is one of the reasons it’s important to detail the specific housing and ventilation type used in disciplines like behavioral research.
“It’s also been shown that one model of IVCs can create a mild hypoxia, which can increase renal vascular conductance and hematocrit hemoglobin concentration of platelet counts, as well as lowering white blood cell counts. So, someone doing immunology research, for example, should understand that the caging system could be a factor,” says Perkins.
Edwin Les developed the first individually ventilated cage in 1979, and Thoren started marketing IVCs in 1980. Since then, the technology has advanced to include a variety of design improvements, ventilation systems, and cage styles made by different manufacturers, but all of them are still generally referred to as IVCs. In order to establish clarity and consistency, Perkins and other researchers advocate the documentation of key parameters for defining IVC systems in manuscripts. These parameters include the manufacturer and model of the equipment, as well as details regarding variables such as air change rates, airflow mechanics, and ventilation design.
“You can get a little bit more information by knowing the manufacturer and model number, if it’s cited in the literature, but you still have to look that up and read all the parameters to understand how it works if you don’t have that system,” says Perkins.
Air Change Rates
Air change rates vary widely among different IVC configurations, and these variables can impact rodent wellbeing. According to the Guide for the Care and Use of Laboratory Animals, the general recommendation for animal room air changes per hour (ACH) is 10 to 15—regardless of how the animals are housed or how the rooms and cages are ventilated—but IVCs can have 50 air changes per hour or more depending on the manufacturer. Air change rates have been shown to impact behavioral research and testing, making it an important consideration in those fields.
“We want to have a good air change per hour within the macroenvironment, but IVCs can have anywhere from 15 to 70. There’s quite a bit of variation and it is possible to have too many air changes per hour. So it’s important to recognize how variations in air changes can impact the research, because air change rates can dilute the pheromones in the cage or even in the room, depending on how the air is exhausted,” says Perkins.
Additionally, ACH calculations are dependent on the volume of material in the cage. There’s a difference between testing the air volume of an empty cage versus the measurement for corrected volume. The corrected volume is based on what else is in the cage, including the number and size of rodents, the amount and type of bedding, and location of food and water dispensers—all of which influence actual air change rates.
“It’s also important to know how the air change rates are determined in the racks. The manufacturer will tell you the number of air changes per hour they have when using blowers, but if you hook it up to your exhaust system you have to figure it out for yourself,” says Perkins.
Airflow Mechanics
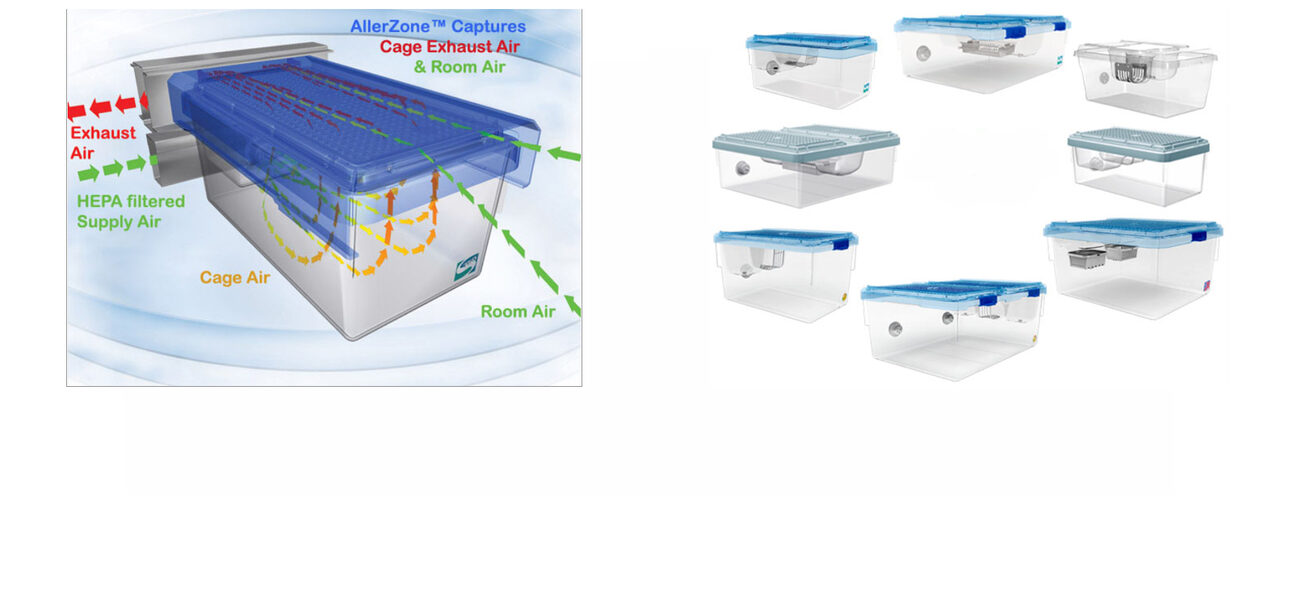
Another factor to consider is the mechanics of airflow design. Some systems have supply and/or exhaust blowers and some have motor-free systems that are connected directly to the HVAC system. Supply air can be delivered into the cage from the top coming down, at the cage level, or up from the bottom of the cage and blow through the bedding; exhaust air can be captured either directly from the cage or indirectly as hood exhaust, all of which can impact the living environment of the animals.
“For example, Thoren basically uses direct airflow, but this is somewhat theoretical in that the supply air is coming in from a hole in the back and exhausting from a hole in the front. All of that, however, is going to depend a little bit on your rodent population, because the animals are going to disrupt that airflow within the cage,” says Perkins.
Some IVC systems have intra-cage air supply with perimeter capture exhaust systems that are very different than direct flow designs. In this configuration, the supply air comes into the cage through a grommet connection and then exhausts via a petri dish-type lid that allows the airflow to be sucked into the plenum exhaust instead of directly from the cage.
“With airflow dynamics, we have to think about the mixing of air in the cages and the purging of exhaust air—whether it’s captured, direct, or convection from the heat of the animals,” says Perkins.
There are also non-sealed cages and specialized sealed cages. Sealed cages are used for containment of high-level biohazards and chemical hazards, but if the system goes down, it can result in rising levels of microenvironmental contaminants, heat, CO2, and other gasses. This means sealed cages should have monitoring systems on them.
Rack Configurations
Rack configurations for IVCs also vary widely. Some systems have supply and exhaust blowers, while others use motor-free ventilated cages. In a motor-free IVC system, the air exhaust of the cage rack is connected directly to the exhaust of the central HVAC and uses negative air pressure to remove contaminants. Studies have shown that mice housed in motor-free IVCs have a greater increase in body weight than those housed in forced-air IVCs, despite similar food consumption. Mice in forced-air IVCs also consumed more water and were frequently located more in the front halves of their cages (possibly due to higher ventilation rates or the location of the air inlets and outlets) whereas mice in motor-free IVCs were dispersed more evenly across their cages. Noise and vibration, both of which can depend on the blower, also have the potential to disrupt breeding and behavior.
“The thing about blowers is that they can be placed on the rack in a variety of different ways,” says Perkins. “Some don’t touch the rack at all, so you’re not getting vibration from the blowers. Others can be on top of the rack. I’ve seen them below the racks on sleds. There’s a variety of different configurations for how blowers attach that may influence the amount of vibration or noise going through the rack. I know of one facility with the same racks in it where some of the blowers are set at 35 air changes per hour and others are set at 70 and you can’t change them. So even within a facility there can be great variation.”
Refined Parameters
According to Perkins and other researchers, there is an increasing need to detail the operating parameters of IVCs in the materials and methods section of scientific publications, instead of just using a generalized acronym and citing the manufacturer and model number. In a letter to the editors of the Journal of the American Association of Laboratory Animal Science, Perkins and Neil Lipman, executive director, Center for Comparative Medicine and Pathology, Memorial Sloan-Kettering Cancer Center, listed these suggested parameters as:
- Airflow mechanics, including how air is supplied to the cage as well as the location of the supply air orifice/diffuser, and how air is exhausted from the cage as well the location of the exhaust orifice.
- Rack ventilation, including the presence of supply and/or exhaust blowers, a description of the rack ventilation if an HVAC system is employed in lieu of a supply and/or exhaust blower, how the rack’s ventilation system is connected to a central ventilation system, and what filtration methods are used, including their type and location.
- Air change rates, including the intra-cage air change rate expressed as the number of air changes per hour (ACH), and how the rate was determined.
- Cage design, including a description of the size and type of filters, the location of the feed container, baffles (if present), and water bottle (size and type), or if the cages use an automatic watering system.
- Intra-cage air flow dynamics, including air velocities and the locations of the measurements, the airflow distribution pattern, and intra-cage air pressure differential (magnitude and direction) with respect to ambient air pressure.
Other variables that could be important to document include the temperature and humidity of the macroenvironment, the type and volume of the bedding and how it was processed (e.g., irradiated or autoclaved), the stock/strain and number of animals per cage, and the biomass (weight) of animals per cage. Even the color of the cages should be documented because it can influence the quality of light the animals experience.
“Clearly defining and documenting these parameters is important from a research perspective, because if someone is trying to reproduce a study, they may not be able to get the same results because their caging system functions completely differently,” says Perkins.
By Johnathon Allen