The unprecedented health and economic impact of the COVID-19 pandemic is driving many institutions to increase investment in new biocontainment facilities or rapidly pivot to upgrade and repurpose existing containment spaces in an urgent attempt to respond to the crisis. Hundreds of organizations nationwide began applying for grants after the National Institutes for Health (NIH) received $3.6 billion in funding dedicated to COVID-19 research as part of an emergency stimulus bill passed earlier this year. The NIH now has until 2024 to release the funds. Additionally, private donors, non-governmental organizations, and other entities worldwide are providing billions in funding for development of testing and vaccine programs. This surge of financing is expected to fuel a growth in the creation of new biocontainment spaces in the near future and long term. Since designing, building, and commissioning new biocontainment labs is an expensive and time-consuming process, some institutions are electing to upgrade existing BSL-2 facilities to make them BSL-3, while others are choosing to move existing research programs to make room for new pandemic-related initiatives.
“In the run-up to the pandemic, there was a dearth of activity in many of the high-containment centers that were built nationally,” says Jeffrey Zynda, science and technology practice leader at Perkins+Will in Boston. “That was primarily due to a lack of funding and the fairly expensive operational costs that come with doing research in these types of facilities. Now, everyone is seeing the future potential of capturing research dollars and grants as being tied to infectious disease research, at least in the short to midterm. So more institutions that may not already have high-containment facilities are clamoring for them.”
In addition to public health labs and regional biocontainment labs funded by the NIH, academic research institutes, pharmaceutical labs, healthcare centers, clinical diagnostic testing centers, and other organizations are restructuring and/or expanding their containment capabilities to be able to work with infectious agents like COVID-19, and create BSL-2 spaces that can be used for doing lower risk work like high-volume diagnostics.
“Obviously, this is something people want to research right now so they can try to get ahead of it and also be a part of something that we can expect to be pretty well-funded in the coming years,” says Ross Ferries, science planner at Flad Architects in Atlanta. “We’re currently working with some institutions that are trying to convert existing spaces to BSL-3 for work on COVID-19. Certainly, there are a lot of different BSL-3 labs out there that were previously doing research on other agents that have now shifted their focus to COVID-19, which means that they will likely be trying to replace facilities for whoever was displaced in the process.”
Rapid Response
One institution that was able to rapidly respond to the crisis was the Louisiana State University (LSU) Health Sciences Center in Shreveport, La., which, in just 12 days, pivoted to adapt existing containment spaces and research expertise to create a high-capacity diagnostic testing facility called the Emerging Viral Threat (EVT) lab. The EVT lab, which opened March 25, was the first facility in north Louisiana approved to conduct COVID-19 testing. In addition to being able to determine if a patient has COVID-19, the lab can screen samples for mutations of the virus and conduct antibody testing.
“When the outbreak hit, some of our top-notch virologists who are internationally recognized reached out to their contacts at the University of Alabama and University of Washington,” says Dr. Chris Kevil, vice chancellor for research at the LSU Health Sciences Center. “On March 12, I got an email saying that they could get the testing protocol to start clinical testing. We had a BSL-3 space and a wing of our research building that we had just made into an open bay concept laboratory that was BSL-2. So I took the idea to the chancellor and said, I think we can do this... Over the course of that afternoon and the next morning, we developed plans to launch the lab. We swiftly got a series of robotics and PCR (polymerase chain reaction) thermocyclers and were able to convert a room into a hot lab in conjunction with our building engineers, so we could take in specimens, inactivate them, and then perform the viral RNA extractions for PCR testing —all within 12 days.”
After starting out testing around 20 to 50 samples a day, the EVT lab ramped up to doing around 8,000 samples per week. Since then, they’ve become the largest contributor of viral genome sequencing for COVID in the state.
“What we’ve learned along the way is that, obviously, when you do something like this, there are a lot of moving parts,” says Kevil. “You’ve got supply chain issues, you’ve got engineering issues, you’ve got containment issues. So it was essential to have everybody on board at the same time. It was really about strategic management and team leadership. One group took care of supply chain and ordering; another group took care of operations of the lab. Another group took care of engineering and making sure the lab actually functioned appropriately.”
Thanks in part to the success of the EVT lab, LSU Health has decided to add an additional floor to the top of a new medical education building slated to break ground in early 2021 that will house another BSL-3 facility along with another BSL-2 compartment for clinical laboratory testing. The added space will allow the institution to handle PCR testing, serology with antibody testing, viral neutralization assays, and next generation sequencing.
“There’s an awareness now at the state and federal level that this is a public health mission, and that we need to have an academic medical center in this part of the country that can pivot and respond quickly to a viral outbreak like we’ve done. That means we really need to be able to maintain a CLIA-certified clinical reference laboratory (certified by the federal Clinical Laboratory Improvement Amendments). And if the need for it decreases, the function of the lab adjusts as needed. But as the need for it expands, we can expand the lab while simultaneously doing experiments that are relevant from a medical and biomedical research standpoint in the meantime.”
Speed, Cost, and Lessons Learned
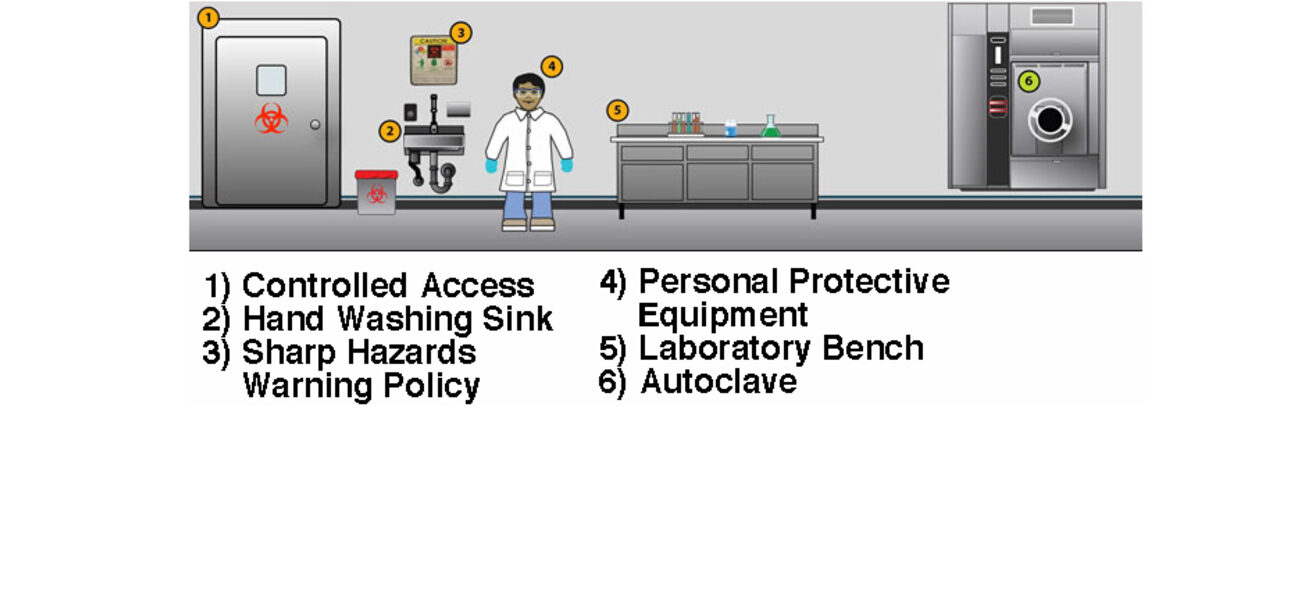
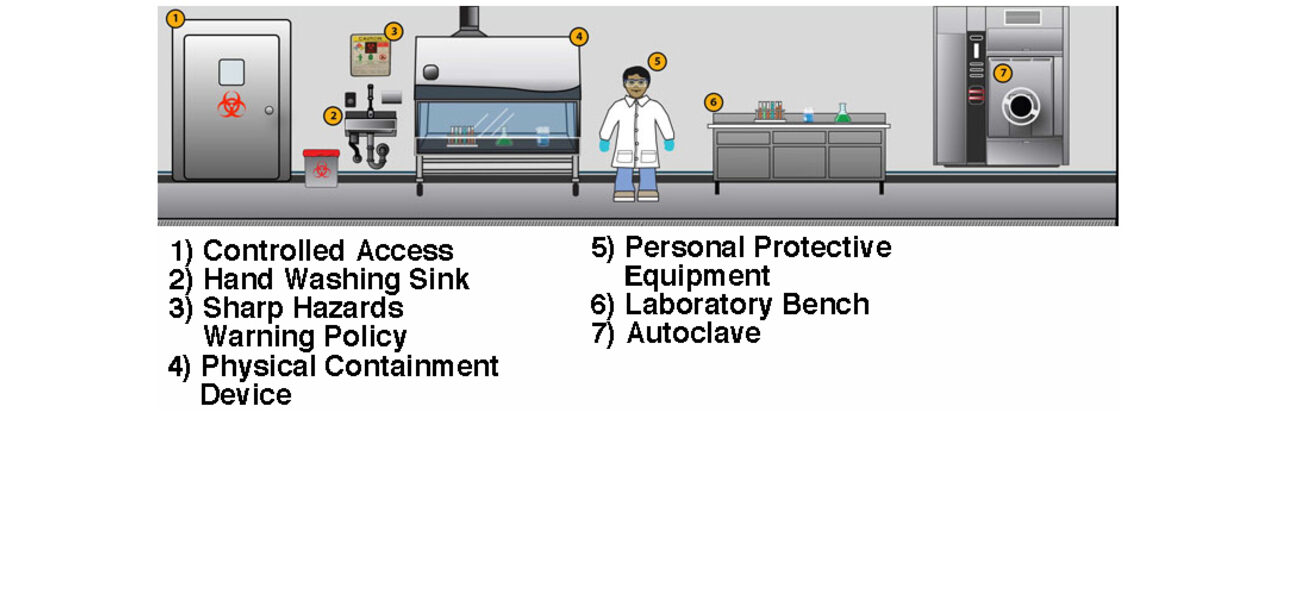
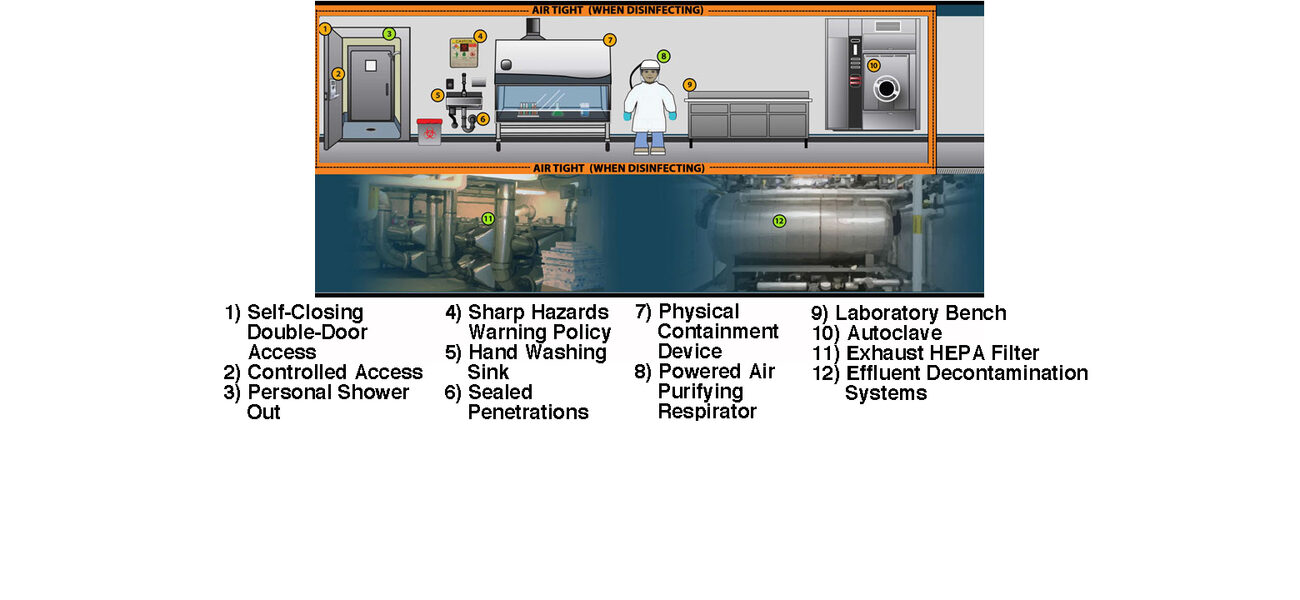
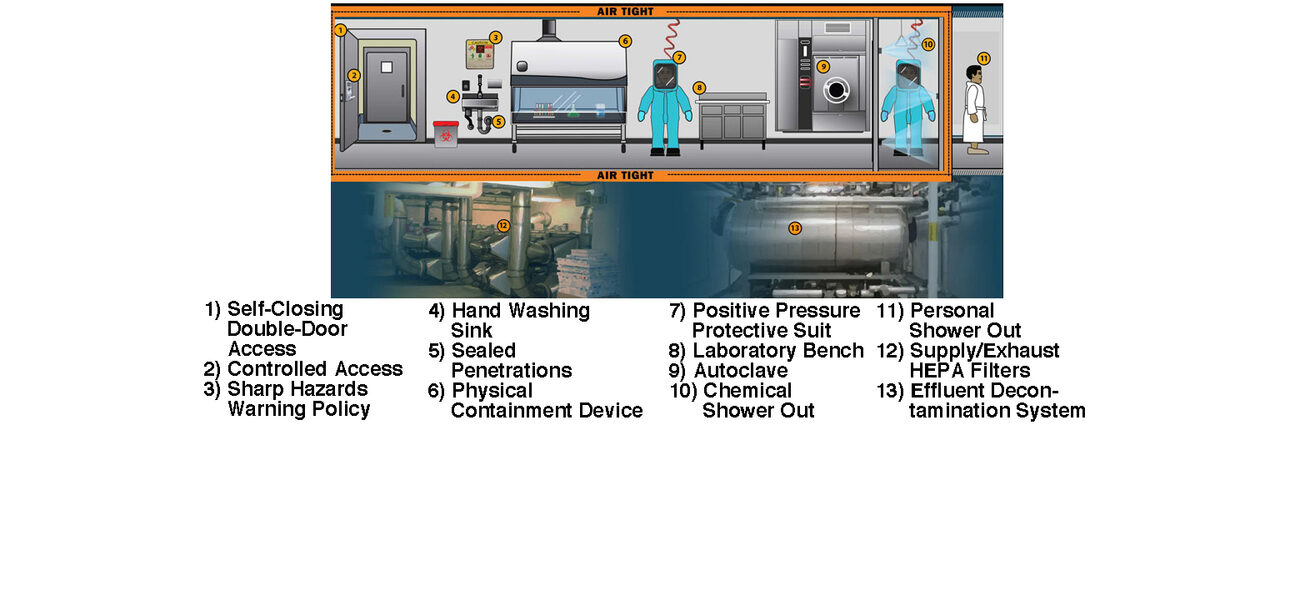
In addition to taking years to design, build, and commission, high-containment facilities are also expensive to staff, operate, and maintain. BSL-3 and BSL-4 facilities require airtight environments with sealed penetrations, once-through air, negative pressure, HEPA filtration, and a highly trained staff, among other features. Despite the fact that the facility design is rigorous, many of the key biosafety measures actually come from the protocols that researchers use while working in the space and how they enter and exit. That said, there are ways to expedite the design and certification process and control costs within certain parameters that include hiring experienced designers and commissioning agents and ensuring the project is flexible and right-sized for the intended mission.
A significant barrier to doing things quickly, however, is a shortage of people who are experienced in running successful high-containment capital projects. In the 10 years from 2002 to 2012, the U.S. and other developed countries built a lot of new high-containment facilities fueled by a wave of government grant funding after 9/11. In the process, they created a cadre of capital project managers at institutions around the world who gained, in many cases through costly mistakes, critical knowledge and lessons learned about design, construction, and capital project management processes that are unique to high containment. “The problem is that over the past nine years, there has been little construction activity for high containment, and many of those people with institutional knowledge about these kinds of capital projects have retired or moved on to other fields,” says Steve Westfall, CEO of Tradeline Incorporated and executive editor of Management Principles for Building and Operating Biocontainment Facilities, which chronicles 10 years of planning and project management lessons learned and identifies 50 unique management principles for containment projects. According to Westfall, not only institutions new to high containment, but even many of those that built facilities more than 15 years ago, are now faced with running high-containment capital projects with inexperienced personnel. “There is not a lot of recorded wisdom out there for building, modifying, or expanding high containment,” says Westfall. “That becomes evident when you Google ‘building biocontainment facilities.’”
“If you’re just doing diagnostics, that can be done in a BSL-2 facility,” says Debra Sharpe, biosafety officer at Cleveland Clinic and president of Sharpe Solutions International. “But if you’re going to be propagating something like the coronavirus, the reality is that you can’t build those types of facilities very quickly. It’s not going to happen within a 12-month window, like many people think. And if you want to test medical countermeasures and therapeutics, you’re going to need animal models. That means you need an ABSL-3 facility, which is even more expensive. There aren’t a lot of ways to fast-track the process, but the first step is hiring architects and engineers who have experience building these facilities and commissioning agents who have lots of experience getting them built and registered.”
“Having an existing research building that has a robust mechanical system is a good start,” says Ferries. “We’re currently working with one group that has an animal research facility, which is inherently the type of construction required. If you have concrete block construction with high-end finishes and once-through air, then it may just be a matter of adding HEPA filtration and handwash sinks in the right place with a few other upgrades.”
“The focus is starting to shift towards what I would call scalable containment, which is a different approach to biocontainment,” says Zynda. “I think the question comes down to whether you’re building a facility that needs to survive for an incredibly long period of time with relatively little to no maintenance, or are you building something that might be around for a year until there’s a significant vaccine breakthrough and then it becomes irrelevant overnight? A lot of colleges and universities have invested in doing COVID testing with light containment as part of their return to campus programs. These aren’t necessarily BSL-3 level containment facilities, but they’re a step towards that. We should take this opportunity to reconsider our standard approaches to biocontainment. If we look to the flexible film isolation technologies that have been the backbone of containment in the U.K. and parts of Southeast Asia, there are some cost-effective ways of creating bio-bubbles or similar approaches to containment in the short-term rather than building an incredibly robust and costly secondary barrier that is the lab itself.”
“Sustainability becomes really critical in these facilities because, while there may be resources available to cover these kinds of operations for the next one, two, maybe three years, the reality is that you have to be diversified enough as an operation to sustain it,” says Kevil. “You’re not going to survive on grant dollars alone. You’ve got to find other revenue streams, whether it’s doing public health outreach and training, providing diagnostics to a CLIA-certified laboratory, or doing genomics and molecular testing.”
Only A Matter of Time
Of course, this isn’t the first time a national crisis has spurred rapid growth in the development of America’s biocontainment capabilities. During the anthrax scare after 9/11, the federal government provided a surge of funding that led to an increase in high-containment facilities that were constructed quickly and are still in place today. But receiving certification by the Centers for Disease Control is a key operating component for doing research with infectious agents. And that takes time.
“A lot of people were surprised by this event, but it wasn’t surprising to those of us who work in the space,” says Sharpe. “Public health professionals like myself have been predicting this for over 30 years. It was just a matter of when. The difference is that most of us thought it was going to be an influenza virus. A coronavirus is not something a lot of people expected. That being said, we expect there will potentially still be an influenza pandemic in the future. Hopefully, we will be better prepared. We’ve certainly had some wakeup calls. I responded to the Ebola outbreak in 2014 when I went to Nigeria and trained 850 healthcare providers. But it seems like every time something like this happens, we start over again like there was no prior history or warning. Certainly, things like NIH funding helps. When there are opportunities to get that grant money, organizations are more incentivized to build these kinds of facilities.”
“I see this having a huge impact over the next 10 years,” says Ferries. “If you look at what happened when there were a few attempted bioterrorist attacks with anthrax after 9/11—that spawned a huge amount funding from the NIH for national and regional biocontainment labs. All from something that didn't really harm that many people or have a huge economic impact. If you think of the number of people that have been infected and the scale of economic impact caused by COVID-19, it’s surely going to change the way we fund public health protection.”
“I think if there is a lesson to be learned from this pandemic, it’s that the specific virus is somewhat irrelevant,” says Zynda. “There will be other pandemics. And that means biocontainment facilities are an investment worth making, especially if it’s going to be one of the key areas of funding moving forward. So we’ve assisted some clients by suggesting that rather than building a large amount of space to handle every plausible scenario, they should scrutinize the amount of containment space they really need. Maybe it’s a pocket system that’s only a hundred square feet for doing the most critical activities. Where you can do other things outside of it through proper risk assessment and management. That way you can build a minimal footprint while being a little smarter about how you spend money and effort. Sometimes, that’s plausible to support the research mission and other times it’s not. But I think we’re starting to see the need for the ability to ramp up containment in a more practically implementable way.”
By Johnathon Allen