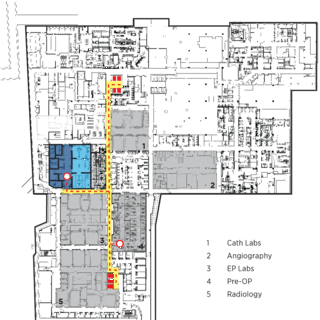
Wexford's @4240 Building
The three-story, 183,000-sf “@4240” building—originally constructed in 1948 as a telephone handset factory—provides flexible tenant solutions, including customized labs for both large- and small-molecule research; dry labs for electronic, medical device, or software research; and modern office space.