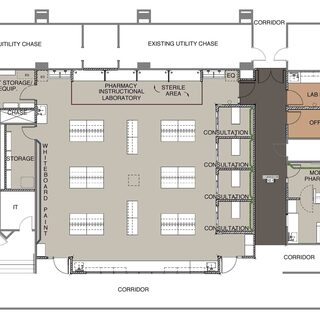
Research and Graduate Education Complex
The four-story, 80,000-sf Research and Graduate Education Complex on the campus of Northeast Ohio Medical University (NEOMED) is a state-of-the-art biomedical research facility for researchers from the colleges of Pharmacy, Medicine, and Graduate Studies. Three floors of laboratories and support spaces house more than 30 scientists and their teams, with space for 156 bench researchers. The building also contains office space for faculty, write-up areas for research staff and students, and small-group teaching and seminar rooms.