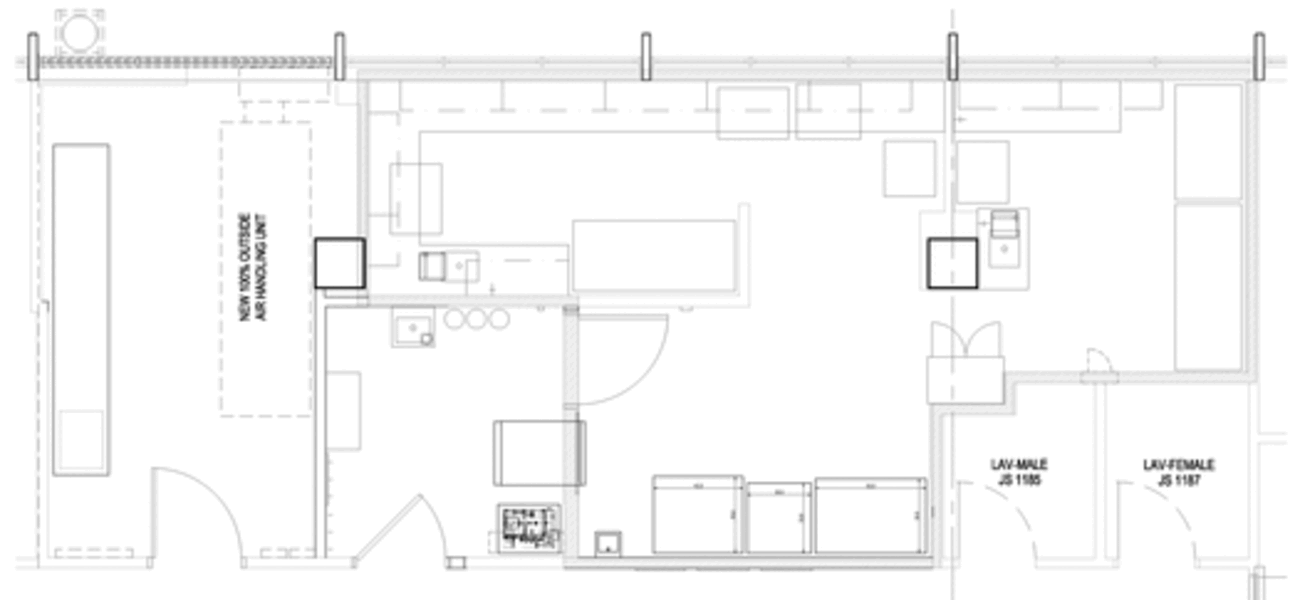
Spurred into action by a broken autoclave, Rush University Medical Center gutted and rebuilt its 30-plus-year-old BSL-3 lab and its anteroom, while converting existing adjacent offices into a dedicated mechanical room. The primary goals with the renovation were to bring the lab up to contemporary BSL-3 standards and to isolate its infrastructure and utilities from the rest of the Jelke Building, a mixed-use lab and patient-care building.
Of key importance was ensuring the separation of the lab’s air systems from the patient population within the building, which includes cardiac catheterization and electro-physiology labs, a blood center, a labor and delivery unit, as well as a neonatal intensive care nursery several floors below. Other tenants in the building include clinical labs that serve the needs of Rush’s patient population across campus in patient rooms and surgery suites via a pneumatic tube system that operates 24/7. Connecting the building’s occupants is a series of shafts that run vertically through the building, carrying building air, power, water, and compressed air throughout different departments, offices, and treatment rooms. The new lab mechanical system ensures that absolutely no supply or exhaust air runs within these existing shafts; instead, the new fully redundant exhaust ducts run directly outside and up the building’s exterior to exhaust HEPA-filtered air well above the building’s roof line.
The renovated lab is used for research by the Virology Quality Assurance Program. As a state-of-the-art Molecular Infectious Disease Core (MIDC) Facility, it conducts research on infectious diseases such as HIV, West Nile Virus, SARS, and pandemic strains of influenza for the National Institutes of Health, and provides specimens to specialty testing facilities across the country.
The Jelke Building was constructed in five stages from 1960 to 1970, and the 493-sf renovated lab, which expanded by approximately 30 sf during the renovation, sits on the 11th floor, in the last of the building stages to be constructed. It is furnished with all new equipment, including an autoclave, two freezers—one minus 80 degrees C and one minus 30 degrees C— a double-door refrigerator, three bio-safety cabinets, and several incubators and centrifuges. The anteroom remains 92 sf, and the new adjacent mechanical space is 205 sf, for a total of 790 gsf.
| Organization | Project Role |
|---|---|
|
Proteus Group Engineers, LLC
|
Architect
|
|
Bulley & Andrews
|
Builder
|
|
Global Biohazard Technologies, Inc. (GBT)
|
Consultant - Biosafety
|
|
Critical Environments Professionals, Inc. (CEPro, Inc.)
|
Consultant - Commissioning
|
|
Proteus Group Engineers, LLC
|
Engineer
|
|
STERIS Corporation
|
Supplier - Autoclaves
|
|
Supplier - Building Automation Controls
|
|
|
Dot Scientific, Inc.
|
Supplier - Centrifuges
|
|
Dot Scientific, Inc.
|
Supplier - Incubators
|
|
Sanyo North America Corp.
|
Supplier - Minus 30 freezer
|
|
Dot Scientific, Inc.
|
Supplier - Minus 80 freezer
|
|
Sanyo North America Corp.
|
Supplier - Refrigerator
|