Building and Fire Codes for Hazardous Chemical Safety: A Primer
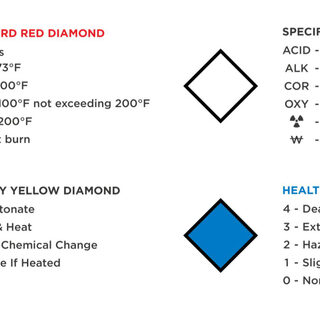
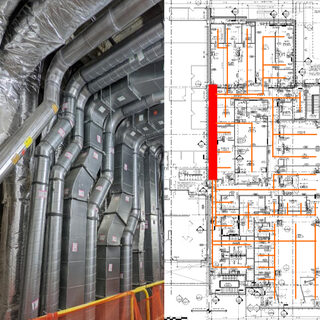
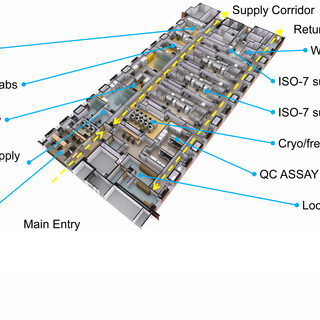
Designing higher education labs is sometimes as much about managing the chemicals that inhabit the space as it is about the scientists and their work in the lab. How hazardous are the chemicals? How are they stored, and what are the allowable quantities? How do you protect the researchers and occupants in adjacent spaces, both during normal lab operations and in an emergency? Is it possible to craft a code-compliant chemical management strategy that aligns with the current shift toward large open labs that foster interdisciplinary collaboration?