Transforming Structure and Culture of Medical Education
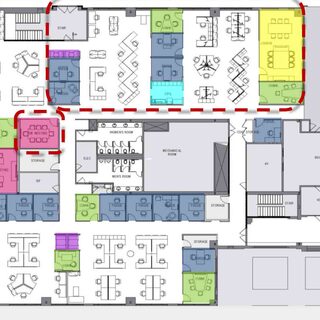
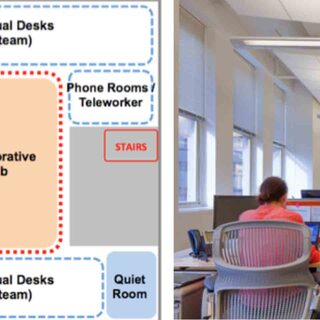
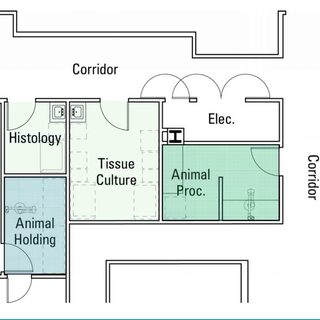
The University of Saskatchewan has renovated and modernized its health sciences education and research facilities to accommodate shared operations, space, and technology, using change management strategies to help faculty, staff, and students move from silos to a team-based model. The transformation incorporates institutional, as well as physical, changes, including restructuring established administrative processes and hierarchies.